Abstract
Introduction:
Over the last several years, non-vitamin k antagonist oral anticoagulant drugs (NOACs) have been developed as a treatment option for prevention of thromboembolic complications in patients with atrial fibrillation (AF). To date, four NOACs have been approved for stroke prevention in nonvalvular atrial fibrillation (AF): apixaban, dabigatran, edoxaban, rivaroxaban Previous studies have indicated that there may be a difference in patient perceptions between these treatment options. However, little is known about the specific preferences of AF patients with regards to NOAC therapy. Therefore, the objective of this study was to elucidate patient preferences regarding attributes that describe the treatment with different NOACs.
Methods:
Patients with AF currently treated with a NOAC therapy were included in a cross-sectional multicentre study in France, Germany and the United Kingdom (UK). Patient preferences were assessed by computer-assisted telephone interviews involving a discrete-choice-experiment (DCE), which is currently seen as gold standard in preference research in healthcare. Within the DCE, each patient needed to decide 8 times between two different hypothetical treatment options, containing 3 convenience-related attributes describing the different treatment options: frequency of intake (once vs. twice daily), size of tablet/capsule (6-9 mm vs. approximately 20 mm) and route of administration (drug must be taken with a meal vs. drug can be taken without or together with a meal). Additionally, one comparator attribute (distance to treating physician: 1 km vs. 15 km) was included in the DCE design. Preferences were analysed based on a conditional logit regression model.
Results:
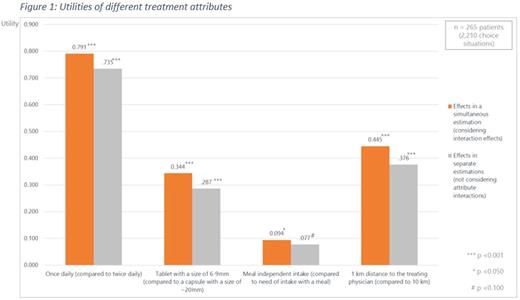
Overall, 327 patients were interviewed. Because of inconsistent responses, 52 patients were excluded (mean age: 71.63 years; females: 41.5%; mean disease duration: 6.33 years; current treatment apixaban/dabigatran/edoxaban/rivaroxaban: 35.1%/24.2%/1.5%/39.2). As presented in Figure 1, the DCE data analysis showed that patients strongly preferred a once daily dosing regimen compared to a twice daily intake frequency (utility: 0.791; p<0.001), a tablet with a size of 6-9 mm compared to a capsule with a size of approximately 20 mm (utility: 0.344; p<0.001) and a distance to the treating physician of 1 km compared to 10 km (utility: 0.445; p<0.001). Also, patients slightly preferred drugs that can be taken independently of a meal (utility: 0.094; p=0.049). In line with this, the attribute ''frequency of intake'' was the most important attribute for a patient's choice (47.2 % of the overall decision) followed by the distance to treating physician (26.6%), the size of tablet/capsule (20.5%), and administration way (5.7%).
Using the applied comparator attribute to translate utilities in a unit that can be easily understood, a patient in the survey was willing to accept a longer distance to the treating physician of 10 miles if therefore treated with a once daily instead of a twice daily dosing regimen. To further confirm these results, more patients will be recruited and included into further analyses.
Conclusions:
The decision of an AF patient for or against a specific NOAC treatment, assuming equal effectiveness and safety of treatments, is mainly driven by the attributes "frequency of intake" and "size of table/capsule". Treating physicians should take patient preferences into account because a treatment that is preferred by patients may also result in an improved adherence and consequently better effectiveness in the real-world environment.
Mueller: Ingress-Health: Employment. Meinecke: Bayer AG: Employment. Buchwald: Bayer AG: Employment. Eriksson: Bayer AG: Employment. Wilke: AstraZeneca: Honoraria; Pfizer: Honoraria; UCB: Honoraria; Eisai: Honoraria; Genentech: Honoraria; Roche: Honoraria; BMS: Honoraria; GSK: Honoraria; Boehringer-Ingelheim: Honoraria; Bayer AG: Honoraria; IPAM: Consultancy, Honoraria, Research Funding; Ingress Health: Employment; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.